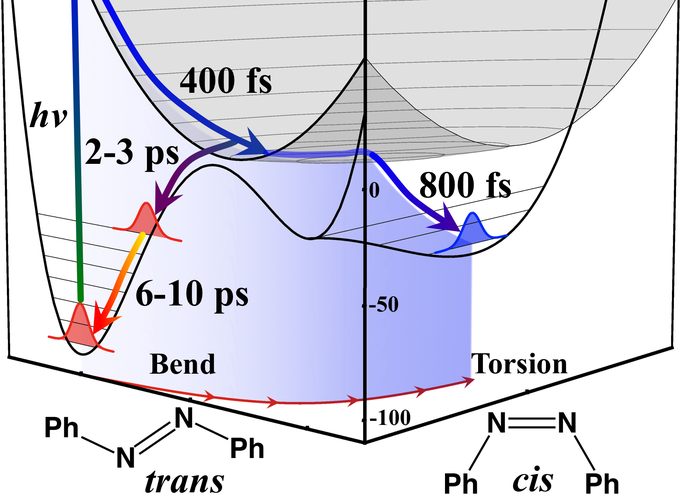

Azobenzenes are used in many applications because of their robust and reversible light induced trans [leftrightharpoons] cis isomerization about the N[double bond, length as m-dash]N bond, but the mechanism of this ultrafast reaction has not been conclusively defined. Addressing this problem we have used Femtosecond Stimulated Raman Spectroscopy (FSRS) to determine the structural transients in the trans → cis photoisomerization of the azobenzene derivative, 4-nitro-4′-dimethylamino-azobenzene (NDAB). Key marker modes, such as the 1570/1590 cm$^{–1}$ NO2 stretch and the 1630 cm$^{–1}$ C–N(Me)2 stretch, enable the separation and analysis of distinct trans and cis photoproduct dynamics revealing the 400 fs Frank-Condon relaxation, the 800 fs timescale of the cis product formation and the 2 ps emergence and 8 ps relaxation of the unsuccessful ground state trans species. Based on these observations, we propose a reaction mechanism, including initial dilation of the CNN bend later joined by quick movement along the CCNN, CNNC and NNCC torsional coordinates that constitutes a mixed inversion-rotation mechanism.