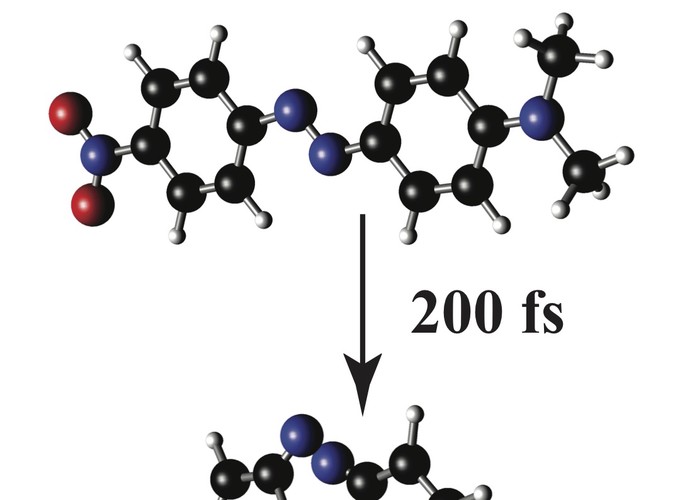

Azobenzenes are versatile photoswitches that find application in optical memory, light-driven motors, and molecular gating. Despite many studies, the molecular details of their light induced trans to cis isomerization are still debated. To inform this discussion we probed the low frequency skeletal motions in an azobenzene derivative, 4-nitro-4′-dimethylamino-azobenzene (NDAB), with resonant impulsive stimulated Raman spectroscopy (RISRS). Four previously unobserved modes at 14, 47, 150, and 201 cm$^{–1}$ were found. Of these, the ∼50 cm$^{–1}$ inversion motion and the ∼15 cm$^{–1}$ torsional motion had particularly large intensities, suggesting that the excited state potential energy surface is steeply sloped along these coordinates in the Franck–Condon region. These data support a model in which NDAB isomerizes predominantly along a prompt inversion coordinate as well as a slower torsional motion that mitigates the phenyl–phenyl interactions on the pathway to the isomerized product.